BORIS is described as: Polynucleotides encoding a nonfunctional mutant form of the Brother of Regulator of Imprinted Sites (BORIS) molecule, nonfunctional mutated BORIS protein, polypeptide or peptide and modified protein forms of BORIS are described. These molecules are used as a therapeutic vaccine against cancer.
CTCFL is a Unique Onco target for Immunotherapy
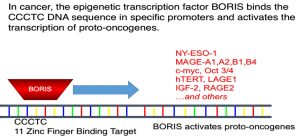
The CTCFL was originally discovered by scientists at the National Institutes of Health (NIH), who demonstrated this protein acts as a critical switch for the initiation and propagation of cancer. CTCFL expression has been shown in many solid tumors including breast cancer. Importantly, CTCFL transforms normal cells to cancer cells, and switching off of CTCFL causes cancer cells to die. CTCFL controls expression of the majority, if not all, cancer-specific antigens used today for clinical trials. For example, it was shown that this cancer stem cell specific oncogene orchestrates MAGE-A, BAG-1, NY-ESO-1 , SBSN, TSP50,FerT, NOTCH3 gene and hTERT. Importantly as mentioned above CTCFL is expressed on cancer stem cells, a small, resilient subset of cells that are central to tumor initiation growth, recurrence and metastasis.
To understand better correlation of CTCFL with cancer we used Big Data analysis-Cosmos site http://catalogue.fiware.org/enablers/bigdata-analysis-cosmos and obtained very important results. More specifically, Big Data analysis of exon sequencing of CTCFL in cancerous and normal tissues showed high mutagenesis rate in various cancer tissues. These mutations occurred in DNA binding ZF-region and in N-and C-terminal regions, indicating the essential role of CTCFL for binding to DNA and survival of tumor cells. These mutations are mostly changing a few amino acids (sometimes one) in protein and may change the binding ability with certain DNA sites. Data from NIH scientists have already shown CTCFL mutations in Wilm’s tumor (pediatric kidney cancer) and these mutations change binding properties of CTCFL to WT1 gene.
These novel data along with published results on immunotherapy suggests that targeting CTCFL represents a paradigm shift in the treatment of cancer. Accordingly based on all this data and based on the fact, that CTCFL expression has been shown in ~70% of primary breast cancer we developed our BRS-001 action plan.
One Approach is BORIS: Universal Cancer vaccine based on Brother of Regulator of Imprinted Sites Molecule
Polynucleotides encoding a nonfunctional mutant form of the Brother of Regulator of Imprinted Sites (BORIS) molecule, nonfunctional mutated BORIS protein, polypeptide or peptide and modified protein forms of BORIS are described. These molecules are used as a therapeutic vaccine against cancer.
What is BORIS?
BORIS = Brother of the Regulator of Imprinted Site
- Discovered by Victor Lobanenkov at NIH
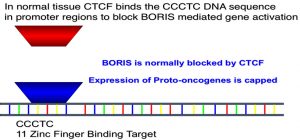
- “Regulator of Imprinted Sites” is CTCF
- CTCF is a tumor suppressor gene
- CTCF is involved in keeping silent parts of DNA that are supposed to be silent
- BORIS is “bad brother” in that it displaces CTCF from DNA, thus allowing for genes that should not be transcribed to be transcribed
“BORIS is a gene that is essential for cancer to be cancer – if a tumor mutates BORIS, then it no longer is a tumor”
– Thomas Ichim, PhD
BORIS Causes Cancer to be Cancer:
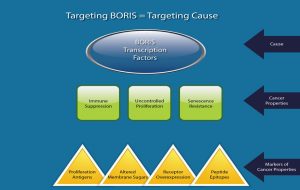
BORIS is Expressed only in Cancer Tissues:
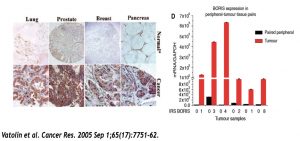
Left Panel: Brown staining indicates the presence of BORIS. As you can see, it only shows up in the bottom rows, which are cancer tissues whereas the top rows are normal tissues from the same patient and organ.
Right Panel: Another way of quantifying BORIS levels. Red bars are from cancer tissues and black bars are from the same tissue, but the non-cancerous part of it.
In Patients Higher Levels of BORIS Equals Lower Survival:

Killing Cancer Stem Cells is Key to Curing Cancer: If you don’t specifically kill the stem cells, the tumor regrows.

BORIS is Found Only in Cancer Stem Cells:

Silencing BORIS Blocks Cancer Stem Cells:

Here, cancer stem cells were prevented from producing BORIS using two different inhibitors (left and middle bars) and they stopped growing. The right bar is a control and those cells kept growing.
How to Kill BORIS? – BRS-001 Cancer Vaccine
BRS-001: Cancer Stem Cell Targeted Immunotherapy
BRS-001 is a patent-pending cellular immunotherapy developed by scientists at Therapeutic Solutions International, Inc., a San Diego Biotechnology Company, which is available at the Pan Am Cancer Treatment Center in Tijuana Mexico to treat stages 1-4 in breast cancer.
BRS-001 activates the immune system to seek and destroy cancer stem cells, based on their expression of a protein named Brother of the Regulator of Imprinted Sites (BORIS). BRS-001 is generated using white blood cells of the patient, which are grown outside of the body to create dendritic cells. The patient’s own dendritic cells are treated in vitro with peptides derived from BORIS, and subsequently are injected back into the patient in order to program the immune response to kill cells that express the protein BORIS, which are cancer stem cells.
Without Killing of Cancer Stem Cells it is Impossible to Cure Cancer
All tumor cells are the offspring of a single, aberrant cell, but they are not all alike. Only a few retain the capacity of the original cell to create an entire tumor. Such cancer stem cells can migrate to other tissues and become fatal metastases. To fully cure a patient’s cancer, it is crucial to find and eliminate all of these cells because any that escape can regenerate the tumor and trigger its spread through the body.
BORIS is Essential for Cancer “To be Cancer”
The BORIS protein functions to disable a tumor suppressor termed “CTCF”[1]. The role of CTCF is to ensure that parts of DNA that should not be activated, indeed are not activated. For example, one of the roles of CTCF is to block expression of genes that cause cancer[2]. In cancer stem cells, BORIS blocks the function of CTCF, thus allowing for propagation of cancer. It has been shown that if BORIS is blocked in cancer stem cells, the cancer stem cells no longer form tumors[3].
BRS-001 is Selective Immunotherapy
Dendritic cells are the most potent immune stimulatory cell of the body. Currently, dendritic cell therapy is approved in the USA in the form of the drug Provenge. BRS-001 consists of dendritic cells that are treated with parts of the BORIS protein in order to stimulate killer T cell responses against any cell that expresses BORIS. Using dendritic cells to stimulate immunity offers the advantage of inducing immunological memory against the tumor. Published studies by us in collaboration with the NIH showed immunity to BORIS results in tumor killing[4],[5].
Preclinical Proof
The BRS-001 construct is capable of stimulating immune responses that cross over to wild-type tumors without having the potential of causing cancer. This ability to induce tumor immunity was validated across a broad variety of tissue types making the BRS-001 approach broadly applicable for numerous cancers. This was described in peer-reviewed papers by Company scientists demonstrating that immunization with BRS-001 not only inhibits growth of aggressive breast cancer 4T1 cells in BALB/c mice, but also that mice immunized with BRS-001 contain high numbers of CD8+ T cells that have spontaneous cytolytic activity against breast, leukemia, and glioma cells in vitro.
The BRS-001 construct is capable of stimulating immune responses that crossover to wild-type tumors without having the potential of causing cancer. This ability to induce tumor immunity was validated across a broad variety of tissue types making the BORIS approach broadly applicable for numerous cancers. This was described in peer-reviewed papers by Company scientists demonstrating that immunization with BRS-001 not only inhibits growth of aggressive breast cancer 4T1 cells in BALB/c mice, but also that mice immunized with BRS-001 contain high numbers of CD8+ T cells that have spontaneous cytolytic activity against breast, leukemia, and glioma cells
invitro.

Company scientists have determined that vaccination with BRS-001 in the context of various immune stimulatory technologies induces a CD8 cytotoxic T cell response that recognizes tumors independent of tissue origin.
NanoStilbene: Patented Augmenter of Cancer ImmunotherapyNanoStilbene, a nanoparticle formulation of pterostilbene, is covered for use in cancer immunotherapy under the Company’s issued U.S. Patent No.: 9,682,047 and is included as part of the Breast Cancer Protocol with StemVacs.
NanoStilbene is an easily absorbed nanoemulsion of nanoparticle pterostilbene in the range of 75-100nm at a concentration of 200 milligrams per milliliter. The pterostilbene placed in a nanoemulsion droplet is free from air, light, and hard environment; therefore, as a delivery system, nanoemulsion can not only improve the bioavailability of pterostilbene but also protect it from oxidation and hydrolysis, while it possesses an ability of sustained release at the same time. Therapeutic uses of nanotechnology typically involve the delivery of small-molecule drugs, peptides, proteins, and nucleic acids. Nanoparticles have advanced pharmacological effects compared with the therapeutic entities they contain. Active intracellular delivery and improved pharmacokinetics and pharmacodynamics of drug nanoparticles depend on various factors, including their size and surface properties. Nanoparticle therapeutics is an emerging treatment modality in cancer and other inflammatory disorders. The National Cancer Institute has recognized nanotechnology as an emerging field with the potential to revolutionize modern medicine for detection, treatment, and prevention of cancer.
Additional StemVacs Platform Immunotherapeutics
Cancer Metabolic DeTox: This is an orally administered agent that is derived from various herbs termed apigenin. The unique property of apigenin is that it inhibits a cancer associated metabolic pathway that degrades the amino acid tryptophan. Specifically, apigenin inhibits the enzyme indolamine 2,3 deoxygenase (IDO), which is responsible for breaking down tryptophan in the vicinity of the tumor and generating by-products such as kynurenine. It is known that immune activation is dependent on tryptophan being present in the tumor environment. The depletion of tryptophan and generation of kynurenine by tumor cells and tumor associated cells is a major cause of immune suppression in cancer. By administering Cancer Metabolic DeTox, the innate arm of the immune system has a chance to regenerate. This positions the patient for better outcome after administration of specific immune stimulating vaccines.
Patent Title: Targeting the Tumor Microenvironment through Nutraceutical Based Immunoadjuvants
Disclosed are compositions useful for the treatment of cancer which modulate tumor associated immunosuppression, thus acting as immunoadjuvants. In one embodiment a composition containing apigenin, is provided, said composition useful for inhibition of tumor associated immune suppression mediated through the molecule indolamine 2,3 deoxygenase (IDO). In another embodiment, liposomal apigenin is administered as a means of decreasing IDO expression.
innaMune: This is a biological product derived from tissue culture of blood cells derived from healthy donors. It is a combination of cytokines that maintain activity of innate immune system cells, as well as having ability to shift M2 macrophages to M1.
Patent Title: Activated Leukocyte Extract for Repair of Innate Immunity in Cancer Patients
Disclosed are compositions, methods of use, and pharmaceutical preparations useful for modulation of immune responses. In one embodiment a composition is extracted polyvalently activated peripheral blood mononuclear cells through dialysis. Said immune modulator is useful for treatment of cancer and alleviation of cancer associated immune depression. In one embodiment, said immunomodulator acts as a costimulatory of T cell activation by modulation of cytokine production. In one embodiment said immune modulator is concentrated for miRNA species capable of activating innate immune cells.
LymphoBoost: LymphoBoost is a proprietary formulation of Mifepristone, a drug approved for another indication, which we have shown to be capable of stimulating lymphocytes, particularly NK cells and T cells, both critical in maintaining anti-tumor immunity.
Augmentation of Anti-Tumor Immunity by Mifepristone and Analogues Thereof
The present invention relates to compositions of matter and methods useful for improving a treatment outcome and/or an alteration of immunity in a condition that benefits from immune stimulation. In particular, one embodiment of the invention teaches administration of sufficient doses of mifepristone or a derivative, alone, or in combination with an immunotherapeutic such as, but not limited to, an antibody, a vaccine, a cytokine, or a medicament whose therapeutic activity is associated with immune modulation.
MemoryMune: This is a product derived from a two-step culture process of donor blood cells. The product MemoryMune reawakens dormant immune memory cells. It is known that many cancer patients possess memory T cells that enter the tumor, however, once inside the tumor these cells are inactivated. MemoryMune contains a unique combination of growth factors specific for immune system cells called “cytokines”.
Patent Title: Methods of Re-Activating Dormant Memory Cells with Anticancer Activity
Disclosed are methods, protocols and compositions of matter useful for stimulation of anticancer immune responses. In one embodiment of the invention culture of buffy coat cells is performed in an environment resembling non-physiological conditions. Buffy coat derived products are subsequently harvested, concentrated, and added to a culture of monocytes and lymphocytes. Conditioned media from said second culture is subsequently utilized as an injectable solution for stimulation of anticancer immunity.
Conclusion: This type of immune response is usually associated with remission of the tumor. Based on this mechanism of action, Therapeutic Solutions International, Inc. has decided to develop a dendritic cell BORIS-peptide pulsed candidate as the most promising method of stimulating immune responses to BORIS in cancer patients. The Pan American Cancer Treatment Center is located a few miles south of sunny San Diego, in Tijuana, Mexico. The Pan Am facilities are state of art and offer access to cutting-edge cancer immunotherapies outside of clinical trials. After we receive you in San Diego, you will travel by air-conditioned transportation to our new and modern treatment center, where you will have access to cellular, small molecule, and protein therapies through accelerated means.
References:
1Hong et al. Cancer Res. 2005 Sep 1;65(17):7763-74.
2Fiorentino et al. J Cell Physiol. 2012 Feb;227(2):479-92.
3Alberti et al. PLoS One. 2015 Jul 17;10(7)
4Loukinov et al. J Cell Biochem. 2006 Aug 1;98(5):1037-43.
5Ghochikyan et al. J Immunol. 2007 Jan 1;178(1):566-73.
